Explain Why There Are Different Isotopes for a Given Element
The number of protons is same in isotopes but number of neutrons is different. They differ in the number of neutrons in the nucleus.
Some isotopes are stable and some are unstable ans can go trough nuclear decay and release radiation.
. B All isotopes of a given element have the same number of protons and electrons. Some elements can only exist in an unstable form for example uranium. The isotope names and symbols are given here.
Stable and unstable radioactive. This type of difference in reactivity likely made it easiest to discover the different isotopes and these differences explain the continued usage of the trivial names even more systematic descriptors are available. Explain why chemical properties are unaffected by a change of isotope.
There are two main types of isotopes. Explain why both symbols have 29 as the bottom number. All atoms of a given element have the same number of protons but some atoms have more neutrons than other atoms of the same element and therefore have a greater mass OR different atomic forms.
Explain why the average atomic mass listed on the periodic table for copper is 6355. The reason for this is because isotopes of an element have the same number of electrons as an atom of that element but they have different number of neutrons which affects the mass number. Different isotopes of a given element have different masses but they have the same chemical properties.
The number of protons is same in isotopes but number of neutrons is different. Explain how they are different. Different isotopes of that element have different numbers of neutrons.
Explain why the solubility of an ionic compound increases as the ionic strength of the solution incr. Chemical properties of different isotopes are almost similar. A Since all the isotopes of an element have identical electronic configuration containing the same number of valence electrons.
The nuclide concept referring to individual nuclear species emphasizes nuclear properties over chemical properties whereas the isotope concept grouping all atoms of each element emphasizes. This difference in neutron amount affects the atomic mass A but not the atomic number Z. Mass number determines the physical properties while atomic number determines the chemical properties.
All artificial lab-made isotopes are unstable and therefore radioactive. Explain what we mean when we say that a particle element consists of several isotopes. This is also the reason why they have different masses.
There are 254 known stable isotopes. So adding a neutron to a nuclear core make it a another isotope by definition. Those variants differ in neutron number and nucleon number.
Isotopes are atoms of the same element that contain different numbers of neutrons. For this reason only certain isotopes are used in nuclear reactions. Isotopes of an element have different physical properties because they have different mass numbers.
The atoms of different isotopes are atoms of the same chemical element. Students also viewed these Modern Physics questions. Isotopes have different masses because they have different numbers of neutrons.
The dramatic differences in mass among the hydrogen isotopes gives the most pronounced isotope effects. All elements have isotopes. Isotopes are different physically to their original atoms.
Isotopes are atoms of the same element that has the same atomic number but different mass number. Isotopes are atoms of an element with the normal number of protons and electrons but different numbers of neutrons. B The fractional atomic masses of elements are due to their isotopes having different masses.
Scientists have found the natural abundances of each isotope. Chemically they behave the same but some isotopes are unstable and will decay. A Isotopes are variants of one chemical element which such as Nitrogen in this case.
This means there is two or more varieties of the same chemical element. Atoms of the same chemical element do not always have the same mass because although the number of protons in the nucleus is the same for all atoms of the same element the number of neutrons is not. Explain how the two isotopes are different from each other.
Any given element is defined by how many protons it has. In a chemical laboratory isotopes of an element appear and react the same. 69 copper-63 and 31 copper-65.
The order of different ionic and molecular species in salicylic acid has to be given question_answer Q. When it comes to physical properties of isotopes including mass melting or boiling point density and freezing point they are all different. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus for example carbon-13 with 6 protons and 7 neutrons.
A isotope is a atom with the same number of protons but different number of neutrons. The charge is determined by the number of protons. For these species the number of electrons and protons remain constant.
Therefore all the isotopes of an element show identical chemical properties. Scientists call them radioisotopes. Isotopes of a given element differ only in the number of neutrons which do not have a charge and thus do not affect the electron configuration.
Isotopes have the same atomic number but different mass numbers.
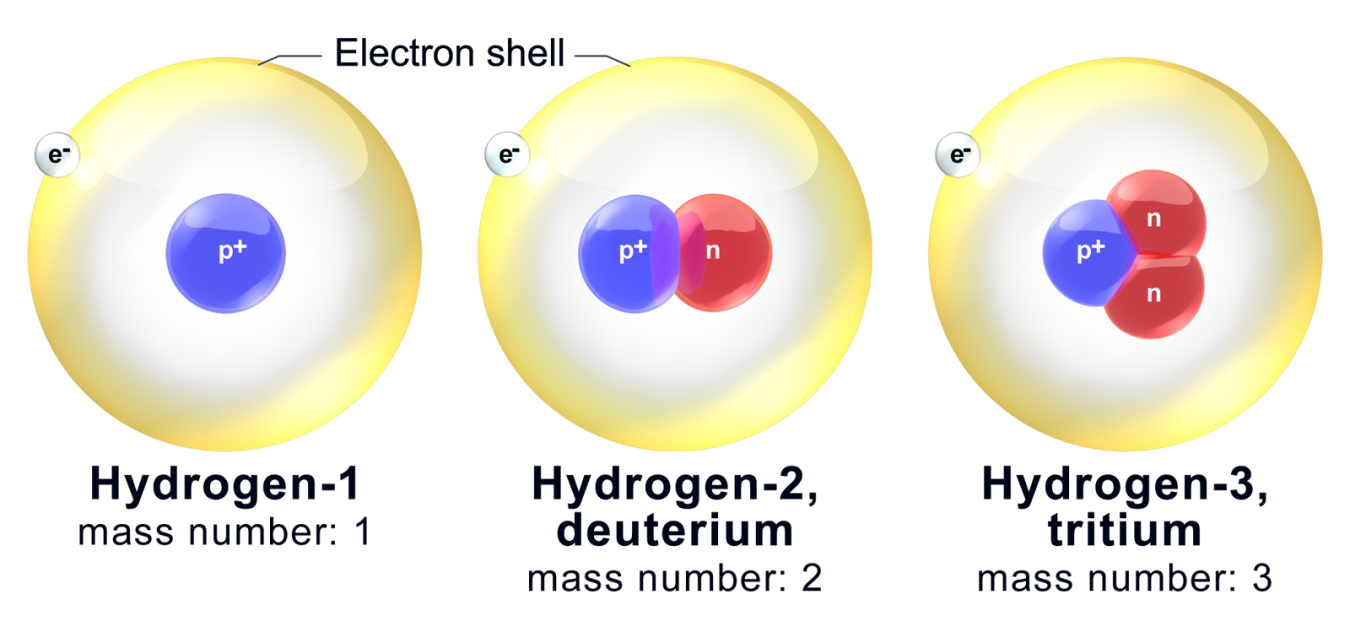
Doe Explains Isotopes Department Of Energy
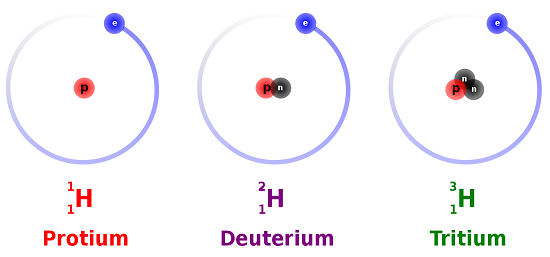
5 8 Isotopes When The Number Of Neutrons Varies Chemistry Libretexts

No comments for "Explain Why There Are Different Isotopes for a Given Element"
Post a Comment